
Colloids
I started my PhD at MIT in September of 2019. USNC was in a state of belated fundraising, and I spent most of my time working on that while just barely getting by in classes. I managed to avoid get suckered into a research project until November, when the apparent slacking started to look egregious, and USNC had finally hit it off with an excellent investor.
The project was on colloids for single-phase heat transfer with Dr. Bren Phillips and Prof Jacopo Buongiorno, as well Abdullah Osman, my friend in his last year as an undergraduate. Later, Minuk Jung, a new graduate student in mechanical engineering, would continue the project. The idea was to define and predict heat transfer performance for colloids in single-phase cooling applications. And then design, make, and test a colloid to maximize that performance. In the end, I learned some plumbing and made observations that did not match predictions. Here's a brief summary of the 100 page report.
Why Colloids?!
Colloids are substances that contain two or more well dispersed components of matter which can be of the same or different phases. Milk is a colloid of fat and protein globs in water. Mayonnaise, paint, and various lubricants are also colloids. Smoke is a colloid made of solid particles in a gas. The size of the particles can vary from micron to nanometer in diameter. Colloids with with nano-sized particles are also called nano-fluids, and these tend to be more stable and less viscous than macro-particle colloids.
During the nanoparticle craze of the 2000s, various dreamy concoctions and questionable measurements suggested there were unusual "nano" effects that gave rise to unexpectedly good thermal hydraulic performance, or the ability to cool down or heat up objects.
With colloids, the hope is that nanoparticles in the fluid will increase thermal conductivity without too seriously degrading the other properties like viscosity, specific heat capacity, and density. Multiple Professors at MIT were hired for that purpose, especially as it applied to nuclear reactor coolants. Nanofluid coolant never really worked out because the fluids would clog or deposit on surfaces and, overall, offered too few, if any, benefits for the added complications. Some of those research projects morphed into nano-patterned surfaces.
Batteries were and continue to receive significant attention for their use in electric vehicles and energy storage more generally. Electric vehicle charging rates are partially constrained by the limited operating temperatures of Li-ion batteries. During charging, heat is generated in the batteries, elevating temperatures, and causing cell degradation or catastrophic thermal runaway. Improved battery thermal management systems (BTMS) can shorten charge and discharge rates and may improve the lifetime and safety of the electrical storage device.
Water is the go-to in cooling applications, but suffers from electrical and corrosion issues. Lubrizol, which sponsored the project, synthesized and characterized colloids for us, and they were particularly interested in organic basefluids with solid particles in suspension. Organic basefluids have reasonable thermal hydraulic properties, but much worse than plain water so something had to be done to close the gap. The approach is then to add nanoparticles to these fluids to make colloids.
A fluid has various properties that affect its ability to transfer heat. In general, better fluids will have higher thermal conductivity, higher specific heat capacity, higher density, and lower viscosity. If we add particles to a basefluid, the hope is that the colloid's properties improve overall. For most fluids and particles of interest, adding particles will degrade performance through an increase in the viscosity (making it more difficult to pump the fluid) and a decrease in the heat capacity. To improve performance overall, these performance losses must be offset by gains in the thermal conductivity and density. The relative importance of these properties depends on the particular cooling problem characterized by the geometry, thermal, and flow conditions.
We use nanoparticles (10-100 nm) instead of larger particles because nanoparticles tend to reduce any erosion, conduction, or stability issues, ensuring the particles don't damage the system, electrically short, or settle out and clog the system. "Nano" also has some serious marketing clout.
The colloids generally look milky and innocuous, sometimes colored dark, and they like to clog up. Nanoparticles have a proclivity to be biologically toxic and the organic basefluids and cleaning fluids are not friendly and probably don't have long term exposure data (hexane, dodecane etc).
Predicting Performance
There are several ways to estimate a colloid's thermo-physical properties using the known basefluid and nanoparticle properties. 1 predict the heat transfer coefficient using Einstein approximation for viscosity, Effective Medium Theory for conductivity, and a volumetric average for heat capacity. In general, we used established methods like these for predicting particle properties.
We developed a new performance metric for heat transfer fluids that bundles up the fluid's thermophysical properties with particular heat transfer problem at hand. It's not enough to look at the heat transfer coefficient or just the fluid's properties. A designer has to weigh the cost and benefits of using a given fluid, which includes various system considerations such as the cost of the fluid, the cost of the pressure boundary which depends on the corrosive properties and pressure of the fluid, the pumping power and pump types needed to achieve a given heat transfer, the allowed temperatures of the heat transfer problem, etc. In the end, we simply compared fluids under the same thermal problem and constraints and measured and predicted just the pumping power. This can be measured with an experimental device and predicted using the thermal properties of the fluid and thermal hydraulic correlations for the geometry at hand.
I built a streamlit applet to look at these things quickly. If the group is still paying the vultr server costs, then it can be found at http://colloid-model.mit.edu.
Designing a Colloid
There's a few parameters we can pick when designing the colloid: the type and size of the particles, the type of fluid, the volume fraction of the particles vs the fluid, and how the colloid is made. We could even use multiple particle sizes and multiple particle types. We just rammed all the possible combinations through the model to find the trends that maximized the performance metric. Gold is too expensive, even though that has the highest gains, and settled with highly available MnO and ZnO. The basefluid design, probably where most of the gains can be made, was out of our hands, and provided by the project sponsor. I suspect the basefluid probably has the biggest impact on performance, with the addition of particles giving slight performance changes.
Measuring Performance
We built a thermal hydraulic loop that uses thermo-couples, IR cameras, flow meters, flow control valves, and pressure transducers to characterize the thermal performance of fluids. It's a lot of fun designing and building this kind of hardware and the rag-tag software to control it.

Results
The whole measurement system boils down to a plot like below, which shows results for three fluids. Pumping power is plotted against the wall temperature of the surface that is being cooled. Everything else in the system is the same across the measurements, be it the geometry or the inlet and outlet conditions. The lower the pumping power at a given temperature, the better the fluid as less energy is used to obtain the same cooling objective (a given temperature on the object or a given heat transfer). A better fluid will have a line further down or to the left. In the below figure, the colloid (purple line, Fluid 475) exhibits lower pumping power across the all the wall temperatures suggesting it is a better fluid than the alternatives shown here.
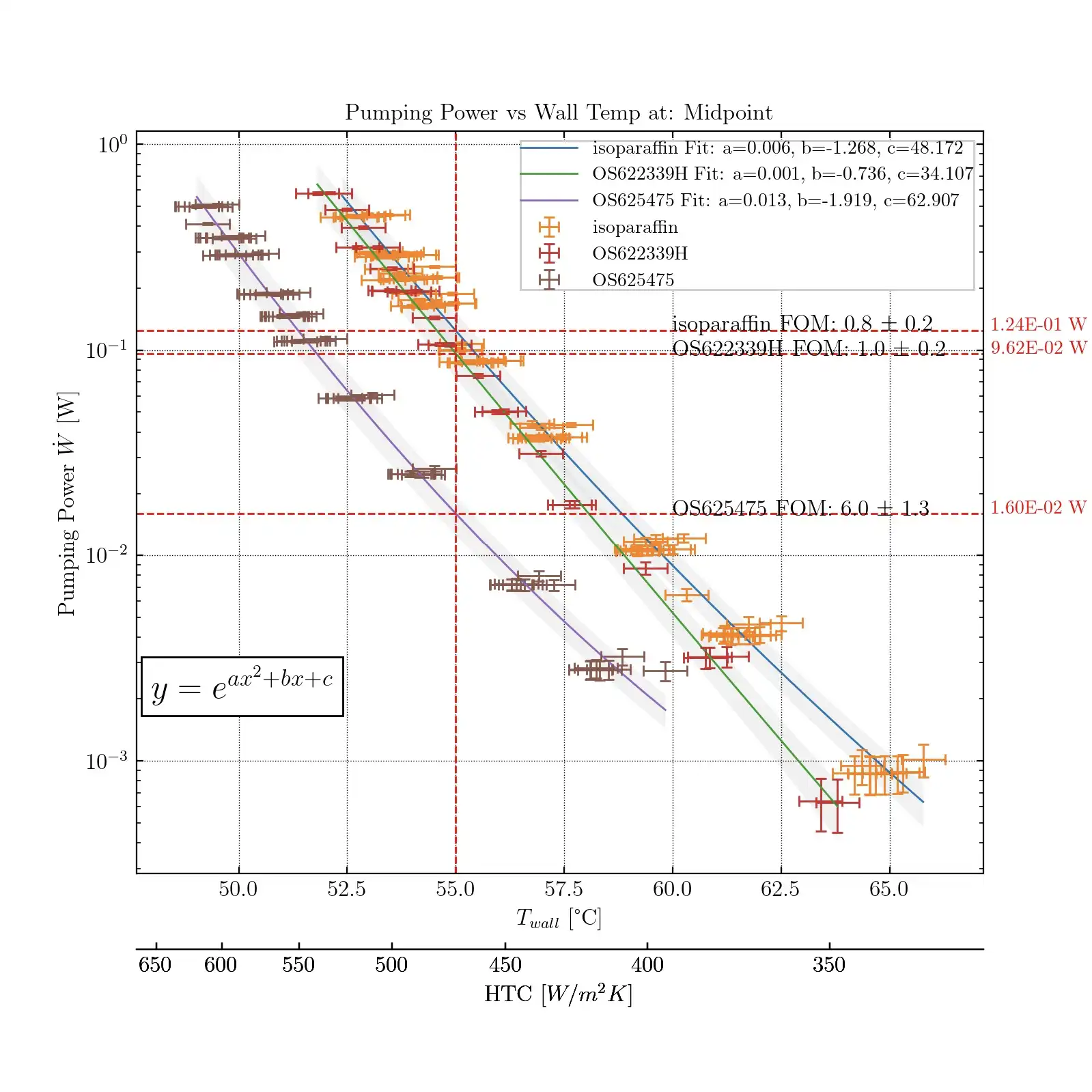
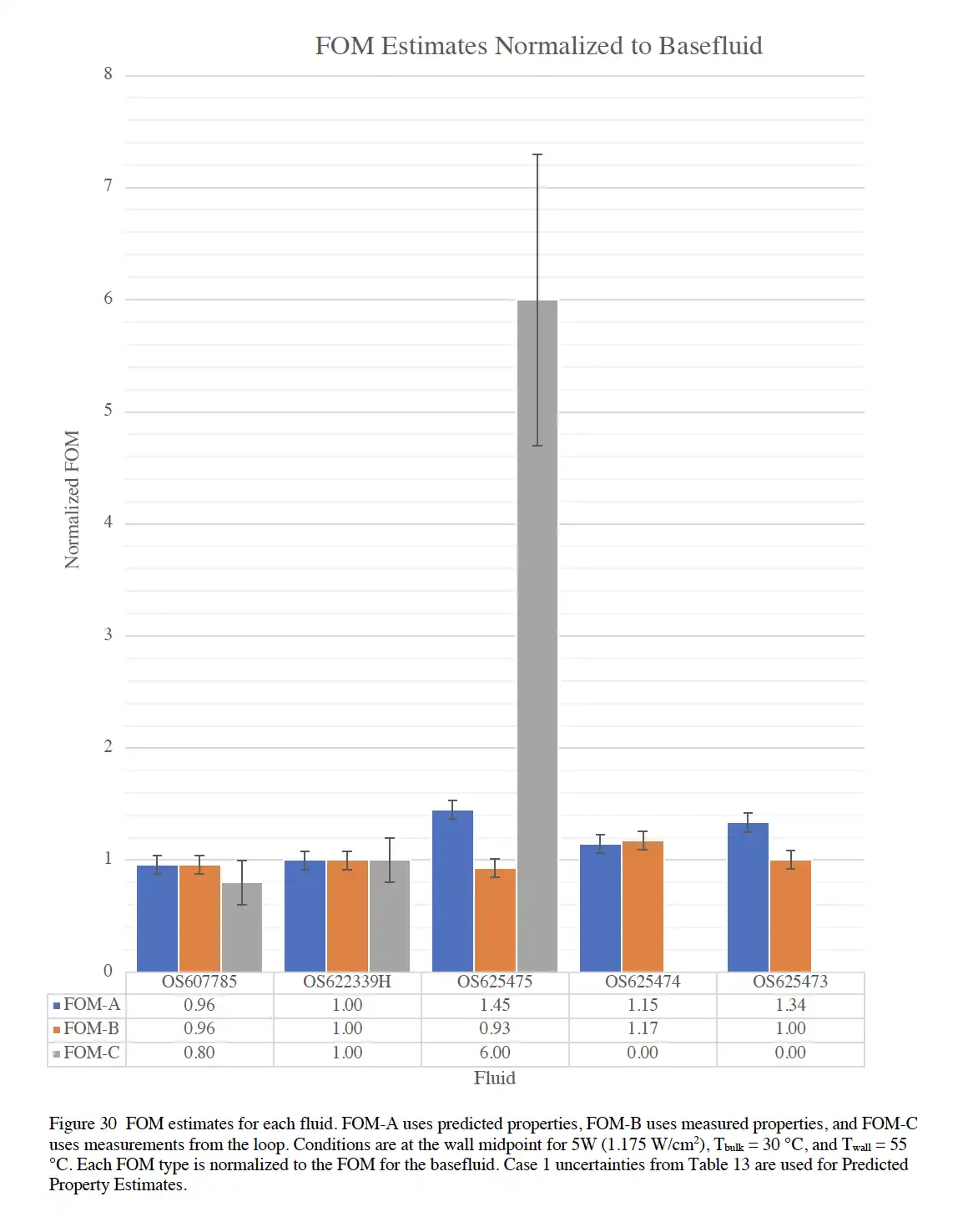
We can simplify the analysis and just look at a vertical line representing a single temperature. This single temperature could be the maximum surface temperature of the battery cell. The colloid (3rd set, Fluid 475) exhibited much better performance than its basefluid (fluid with no particles) and reference fluid. The problem is that the improved performance (grey bars) is much larger than we predicted using the fluid's thermophysical properties and thermal-hydraulic correlations (blue and orange).
I spent a few weeks trying to come up with and disprove various explantations on how either the predictions or the measurements were way off. In the end, I wrote up the report leaning towards the idea that the measurements were sound while the predictions suffered from inaccuracies owing to the property measurements and use of incompatible thermal-hydraulic correlations that did not sufficiently account for the particular geometry and natural circulation. I then jumped ship and moved to a new project.
Notes
Footnotes
-
[@NaveenPrabhat2012, Convective heat transfer enhancement in nanofluids: Real anomaly or analysis artifact?] ↩